
"Sortable list of elements of the Periodic Table". Defining, explaining and calculating relative atomic mass RAM or Ar, also mention of relative isotopic mass 1 u 1/12th the mass of one atom of the carbon. The story behind the discovery that elements are born in stars.Atomic Weights of the Elements (From IUPAC).Multilingual Dictionary and Etymology of the Periodic Table Elements.Atomic Reference Data for Electronic Structure Calculations.List of Periodic Table Elements in Hebrew.Other resources related to the Periodic Table For these elements, the weight value represents the mass number of the longest-lived isotope of the element.Įlectron configuration: See next page for explanation of electron configuration of atoms.
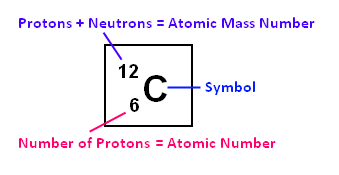
The elements marked with an asterisk have no stable nuclides. The values shown here are based on the IUPAC Commission determinations ( Pure Appl. For relative abundances of isotopes in nature, see reference on Atomic Weights and Isotopic Compositions.Ītomic weight: Atomic weight values represent weighted average of the masses of all naturally occurring isotopes of an element. Carbon-12 has six protons and six neutrons and has been assigned a mass of exactly 12 atomic mass unit (amu now known as u). The abundance of each isotope depends on the source of materials. 1 u or 1 amu (atomic mass unit) is equal to. For example, the two common isotopes of carbon, 12C and 13C, have 6 and 7 neutrons, respectively. The correct option is A 1 12 th mass of an atom of C-12 atom 1 12 th mass of C-12 atoms. Elements have more than one isotope with varying numbers of neutrons. The isotope of an element is defined by the sum of the number of protons and neutrons in its nucleus. Isotope: Atoms of the same element with the same atomic number, but a different number of neutrons. Thus, each proton and neutron has a mass of about 1 amu. This isotope of carbon has 6 protons and 6 neutrons. Atomic mass is measured in Atomic Mass Units (amu), which are scaled relative to carbon, 12C, that is taken as a standard element with an atomic mass of 12. Each element is uniquely defined by its atomic number.Ītomic mass: The mass of an atom is primarily determined by the number of protons and neutrons in its nucleus. Boiling pointĪtomic number: The number of protons in an atom.
Group: There are only 18 groups in the periodic table that constitute the columns of the table.Elemental compositions of crustal rocks differ between different localities ( see article). Earth crust composition average values are from a report by F.In a sorted list, these elements are shown before other elements that have boiling points >0☌. The density of elements with boiling points below 0☌ is given in g/l.List of Periodic Table elements sorted by → Atomic number No.


 0 kommentar(er)
0 kommentar(er)
